Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which kind of graph would be best to use with these data? M&M’s™ in a bag
| Color | Orange | Brown | Yellow | Green | Blue | Red | | Percent | 20 | 13 | 14 | 16 | 24 | 13 | | | | | | | |
a. | Line graph | c. | Bar graph | b. | Circle graph | d. | None of the
above |
|
|
|
2.
|
Which kind of graph would be best to use with these data? | Day of the Week | Monday | Tuesday | Wednesday | Thursday | Friday | | Table Monitor | Maria | James | Tyler | Ashley | Daniel | | | | | | |
a. | Line graph | c. | Bar graph | b. | Circle graph | d. | None of the
above |
|
|
|
3.
|
Which kind of graph would be best to use with these data? | Amount of Salt added | 0 g | 2 g | 5 g | 10 g | 15 g | | Boiling temperature, °C | 99.7 | 101.2 | 102.4 | 106.9 | 108.3 | | | | | | |
a. | Line graph | c. | Bar graph | b. | Circle graph | d. | None of the
above |
|
|
|
4.
|
Find the slope of the line. 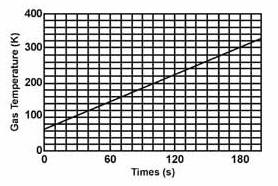 a. | 1.30 | c. | 1.22 | b. | 1.67 | d. | 0.77 |
|
|
|
5.
|
Which is the correct measurement for location marked by the arrow? 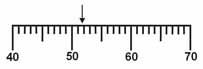
|
|
|
6.
|
Which is the correct measurement for location marked by the arrow? 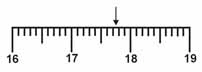 a. | 17.076 | c. | 17.76 | b. | 17.8 | d. | 17.7 |
|
|
|
7.
|
Which is the correct measurement for location marked by the arrow? 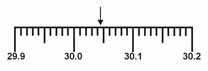 a. | 30.5 | c. | 30.45 | b. | 34.5 | d. | 30.046 |
|
|
|
8.
|
The accepted value is 29.35. Which correctly describes this student’s
experimental data? | Trial | Measurement | | 1 | 29.48 | | 2 | 28.97 | | 3 | 29.27 | | |
a. | accurate but not precise | c. | both accurate and
precise | b. | precise but not accurate | d. | neither accurate nor precise |
|
|
|
9.
|
The accepted value is 1.43. Which correctly describes this student’s
experimental data? | Trial | Measurement | | 1 | 1.29 | | 2 | 1.93 | | 3 | 0.88 | | |
a. | accurate but not precise | c. | both accurate and
precise | b. | precise but not accurate | d. | neither accurate nor precise |
|
|
|
10.
|
The accepted value is 15.63. Which correctly describes this student’s
experimental data? | Trial | Measurement | | 1 | 12.84 | | 2 | 13.02 | | 3 | 12.96 | | |
a. | accurate but not precise | c. | both accurate and
precise | b. | precise but not accurate | d. | neither accurate nor precise |
|
|
|
11.
|
Which is the term used for the measurement of the average kinetic energy of the
particles of a substance?
a. | density | c. | length | b. | temperature | d. | mass |
|
|
|
12.
|
Which unit is determined by the frequency of the radiation given off by
Cesium-133?
a. | meter | c. | hour | b. | second | d. | gram |
|
|
|
13.
|
Which term is described as the amount of matter in an object?
a. | density | c. | mass | b. | volume | d. | length |
|
|
|
14.
|
Which is NOT an appropriate unit for measuring density?
|
|
|
15.
|
Which term describes the amount of mass contained per unit volume?
a. | mass | c. | length | b. | temperature | d. | density |
|
|
|
16.
|
An object has a mass of 26.94 grams and a volume of 2.568 cubic centimeters.
What material is it likely to be made of? | Substance | iron | gold | silver | copper | | Density
(g/cm3) | 7.874 | 19.32 | 10.49 | 8.92 | | | | | |
a. | Iron | c. | Silver | b. | Gold | d. | Copper |
|
|
|
17.
|
Which value has only 4 significant digits?
a. | 6.930 | c. | 8450 | b. | 0.0450 | d. | 0.392 |
|
|
|
18.
|
The accepted value for the density of iron is 7.87 g/cm3. A student
records the mass of a 20.00-cm3 block of iron as 153.8 grams. What is the percent error
for the density measurement?
a. | 19.4% | c. | 2.29% | b. | 7.69% | d. | 2.34% |
|
|
|
19.
|
The accepted value for a quantity is 5.67 g. If the measured value is 5.89 g,
what is the percent error?
a. | 1.04% | c. | 3.74% | b. | 3.74% | d. | 3.88% |
|
|
|
20.
|
Which is the correct value for the prefix deci-?
a. | one-tenth | c. | hundred | b. | ten | d. | one-thousandth |
|
|
|
21.
|
Which is the value associated with the prefix nano-?
a. | one-millionth | c. | one-thousandth | b. | one-billionth | d. | billion |
|
|
|
22.
|
Which is the correct conversion for a temperature of 67.3°C?
a. | -205.7 K | c. | 153.1° F | b. | 121.1° F | d. | 19.6° F |
|
|
|
23.
|
Which is the sum of these values, to the appropriate number of significant
digits?
17.358 + 3.502 + 20.14 =
a. | 41 | c. | 41.000 | b. | 41.00 | d. | 41.0 |
|
|
|
24.
|
Which is the product of these numbers, to the appropriate number of significant
digits?
56.2 ´ 9.2057 =
a. | 517 | c. | 517.36 | b. | 517.4 | d. | 517.00 |
|
|
|
25.
|
How many significant digits are in the value 530,405,000?
|
|
|
26.
|
How many significant digits are in the value 0.0050340?
|
|
|
27.
|
A bottle contains 3.100 mL of a liquid. The total mass of the bottle and the
liquid together is 6.300 g. The mass of the empty bottle is 4.240 g. What is the density of the
liquid?
a. | 0.665 g/mL | c. | 1.505 g/mL | b. | 1.368 g/mL | d. | 2.032 g/mL |
|
|
|
28.
|
What is the distance that light travels through a vacuum in 1/299 792 458 of a
second?
a. | centimeter | c. | meter | b. | kilometer | d. | nanometer |
|
|
|
29.
|
According to rounding rules for addition, the sum of 27.1, 34.538, and 37.68 is
____.
a. | 99.318 | c. | 99.31 | b. | 99.3 | d. | 99 |
|
|
|
30.
|
Calculate the slope of the line in the graph. 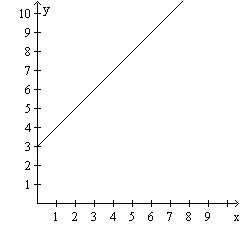
|
|
|
31.
|
The graph represents the sales data for a particular product. In which week were
8 units sold? 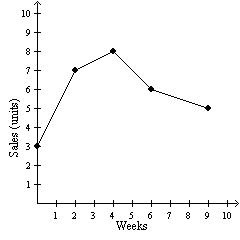 a. | 3rd week | c. | 6th week | b. | 4th week | d. | 9th
week |
|
|
|
32.
|
The product of 2 ´ 104 cm and 4 ´ 10–12 cm, expressed in scientific notation is
____.
a. | 8 ´ 10–7 cm | c. | 8 ´ 10–8 cm | b. | 6 ´
10–8 cm | d. | 8
´ 10–48 cm |
|